PhD
Did you ever throw too much sugar in your coffee mug? Know the feeling? If you are quick, you can still try to fish it out, but once dissolved, it is almost impossible to remove the excess sugar. Why? The bigger sugar cubes broke down into different small building blocks: the individual sugar molecules. And those are floating in your coffee. Fishing them out with a coffee spoon will not work.
During my PhD, I studied a novel method which is capable of removing such tine molecules. The focus did not lie on the removal of sugar from coffee, but in the removal of a chemical with the name butanol. Butanol is an extremely important chemical building block, which serves as a component to make rubber or paint. We make all our butanol from petroleum. But scientists discovered bacteria who are capable to make butanol from green, organic waste. Examples are straw, wood or anything you can compost: a renewable, green source for this important building block.
Unfortunately, this butanol ends up in a soup of bacteria and waste. Just like removing the sugar from your coffee, butanol is extremely difficult to capture. During my PhD, I used materials which behave like a sponge: they contain of extremely tiny pores and are capable to remove butanol from this soup of waste and bacteria.

Butanol directly from this soup of waste and bacteria
In the ideal case, we remove butanol directly from this mixture of organic waste and bacteria. And this is possible. We discovered some commercial materials which can remove butanol very selectively from such a mixture. We could also reuse these materials: by heating them, butanol evaporates from the pores.
Butanol from fermentation vapors
The bacteria we use also produce a lot of gas. This mixture of waste and bacteria therefore looks a lot like a bubbling glass of champagne. Part of our butanol evaporates from our soups through these bubbles. We investigated whether we could use our adsorbents also to remove butanol from these gases. Result? Materials that worked well to remove butanol directly from the liquid mixture, were less suited. Researcher in Valencia, however, could make a completely new material. This adsorbent proved to be extremely well-suited to remove butanol from such vapors.
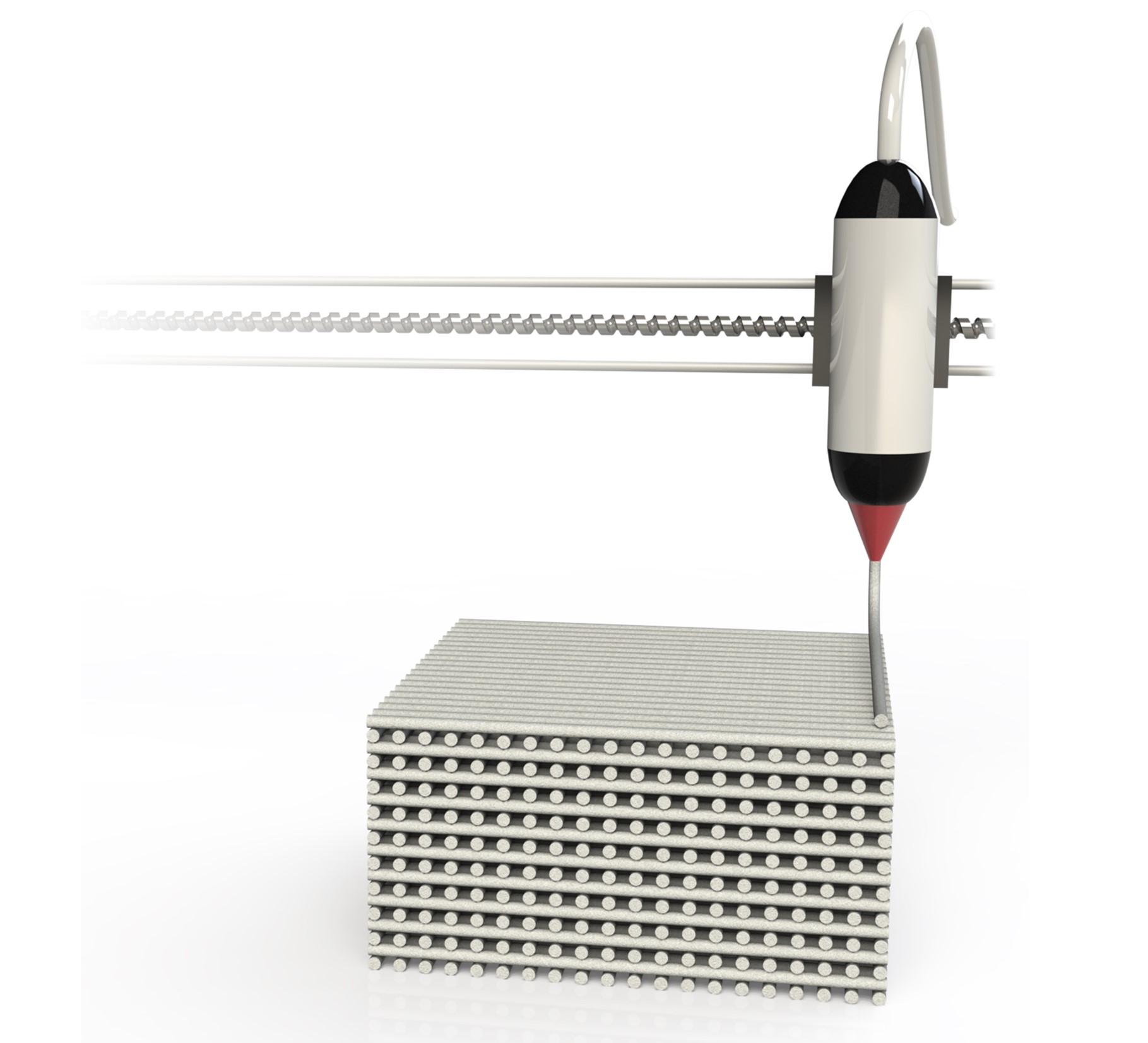
3D-printed adsorbents
In a different collaboration, together with researchers of the Vlaams Instituut voor Technologisch Onderzoek (VITO), we investigated the use of adsorbents which were 3D-printed. The amounts of gas produced are extremely large. Normally, we put spherical beads of adsorbent of around 1 mm into a cylindrical tube. If we have to treat such large amounts of gas, this leads to trouble: the pressure needed to push our gas through such a tube becomes excessively large. We could print new adsorbent shapes which solve such problems. On top of that, the design of these structured proved to have a major influence in how the gas flows through our column.
Hugging molecules
Wat does actually happen in those tiny pores of our adsorbents? Why do certain materials work and other do not? These questions we tried to understand in the final parts of my PhD. We discovered in one adsorbent that butanol is attracted by other molecules in the mixture. Due to this effect, butanol suddenly adsorbs much stronger. We could exploit this effect to recover butanol at higher concentrations.